+86 10-53676895
1368690224@qq.com
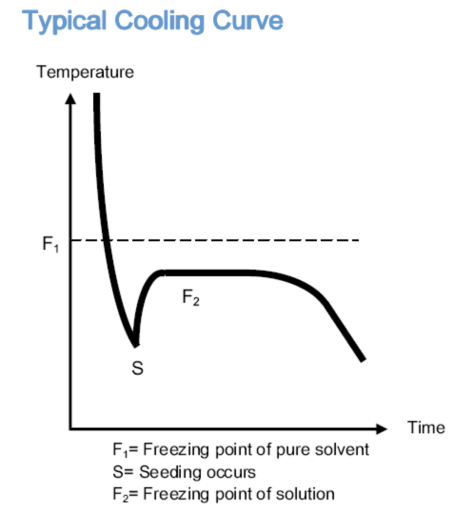
PSI CRYETTEThe freezing point meter uses supercooling and thermocouples to accurately measure the freezing point of a solution. The supercooling phenomenon refers to the phenomenon where the temperature of a solution is below the freezing point without freezing. When a supercooled solution is stirred, the solution crystallizes. After crystallization, the solution will release a certain amount of heat, which is called crystallization heat. As shown in the figure below, the solution is supercooled and at the freezing point (F1)The following is still liquid and will crystallize when shaken or stirred(S)The released crystallization heat causes the temperature of the solution to rise. The temperature of the sample rises to the freezing point platform (F2); Afterwards, the temperature continued to drop. The temperature of the platform is the freezing point of the solution.
Contact below if you would like to get a reply quicker.